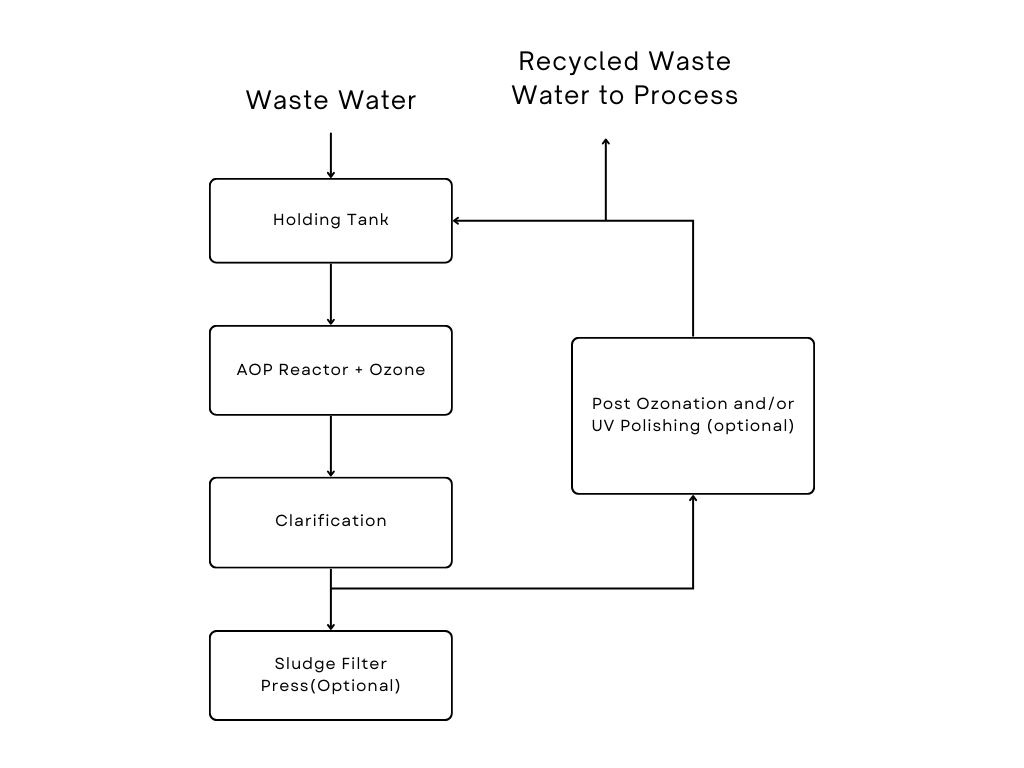
Advanced Oxidation Process
AOPs rely on in-situ production of highly reactive hydroxyl radicals (·OH). These reactive species are the strongest oxidants that can be applied in water and can virtually oxidize any compound present in the water matrix, often at a diffusion controlled reaction speed. Consequently, ·OH reacts unselectively once formed and contaminants will be quickly and efficiently fragmented and converted into small inorganic molecules. Hydroxyl radicals are produced with the help of one or more primary oxidants (e.g. ozone, hydrogen peroxide, oxygen) and/or energy sources (e.g. ultraviolet light) or catalysts (e.g. titanium dioxide). Precise, pre-programmed dosages, sequences and combinations of these reagents are applied in order to obtain a maximum •OH yield. In general, when applied in properly tuned conditions, AOPs can reduce the concentration of contaminants from several-hundreds of ppm to less than 5 ppb and therefore significantly bring COD and TOC down, which earned it the credit of “water treatment processes of the 21st century”.
The AOP procedure is particularly useful for cleaning biologically toxic or non-degradable materials such as aromatics, pesticides, petroleum constituents, and volatile organic compounds (VOC) in waste water. The contaminant materials are converted to a large extent into stable inorganic compounds such as water, carbon dioxide and salts, i.e. they undergo mineralization. A goal of the waste water purification by means of AOP procedures is the reduction of the chemical contaminants and the toxicity to such an extent that the cleaned waste water may be reintroduced into receiving streams.
AOP Purpose:
The AOP (Advanced Oxidation Processes) is usually used for removing contaminants from waste water coming out of several types of heavy industries like:
- Petrochemical & Plastic Industry
- Chemical Industry.
- Food Processing Industry
- Pharmaceutical Industry
- Metal and Metal Plating Industry
- Textile and Dying Industry
- RO & NF brine streams
Utilizing a Range of Oxidants for Maximum Efficiency
Advanced chemical oxidation processes make use of (chemical) oxidants to reduce COD/BOD levels, and to remove both organic and oxidisable inorganic components. The processes can completely oxidize organic materials to carbon dioxide and water. AST is using a wide variety of advanced oxidation processes.
Chemical oxidation processes using hydrogen peroxide, ozone, combined ozone & peroxide CLO2 Ultra Sound & Ultra-violet enhanced oxidation such as US/UV/ozone, US/UV/hydrogen peroxide, US/UV/air and catalytic reagent. Advanced Oxidation Processes are particularly appropriate for effluents containing refractory, toxic or non-biodegradable materials.
The processes offer several advantages over biological or physical processes, including:
- Process operability(No biological process)
- Unattended operation with very small foot print.
- The absence of secondary wastes (sludge).
- The ability to handle fluctuating flow rates and compositions.
